New alopecia treatment CTP-543 helps hair regrowth in alopecia areata patients, possible cure?
Clinical Trial in Alopecia Areata
What CTP-543?
CTP-543 is an investigational oral selective inhibitor of Janus kinases JAK1 and JAK2. The FDA has granted CTP-543 Breakthrough Therapy designation for the treatment of adult patients with moderate to severe alopecia areata and Fast Track designation for the treatment of alopecia areata and could be a potential cure for Alopecia areata.
Significantly more trial participants who received Concert’s drug, called CTP-543, experienced greater hair regrowth following treatment than did those who were given a placebo in the study. The effect held up across both a lower and a higher dose of CTP-543, which also met the trial’s secondary goals.Concert’s trial, which enrolled about 700 adults with moderate-to-severe alopecia areata, is one of two Phase 3 studies testing the drug. Results from the second are expected in the third quarter, and Concert anticipates the combined data will form the basis of an approval application to the Food and Drug Administration.
CTP-543 release date: Concert will submit the study’s full results for publication in a medical journal. If Concert’s second study succeeds as well, the company plans to submit for FDA approval in the first half of 2023.
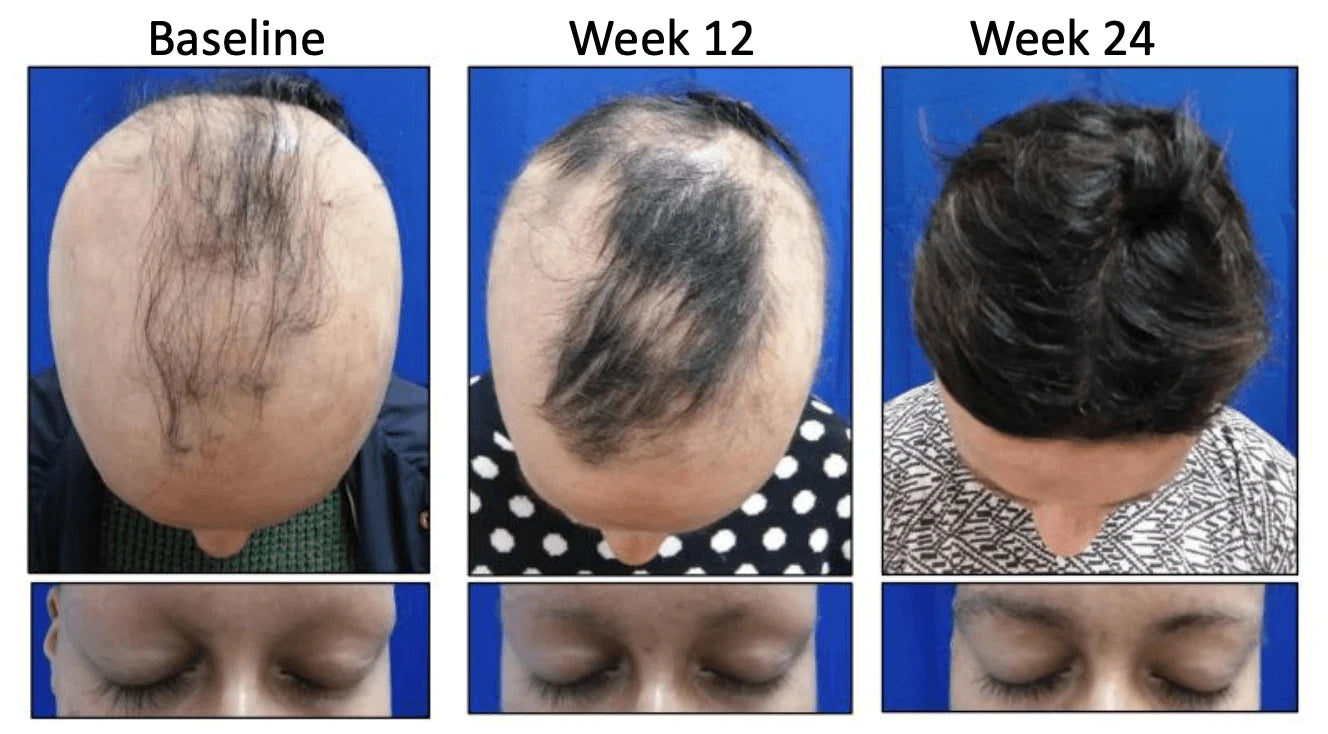
Researchers at Yale University, Connecticut, found Concert Pharmaceuticals' experimental drug CTP-543 helped patients with alopecia areata regrow nearly a full head of hair. Pictured: A patient in previous Phase 2 clinical trials at the start (left) and end (right) of the 24-week study

Statistically Significant Hair Regrowth Observed as Early as Eight Weeks CTP-543 Has Potential to be Best-in-Class for the Treatment of Alopecia Areata
We cant wait until this is out to help our Watermans customers.


















